Capecitabine-Induced Terminal Ileitis: Review of the Literature After a Case Report
DOI:
https://doi.org/10.60591/crspmi.39Keywords:
Capecitabine/adverse effects, Ileitis/chemically inducedAbstract
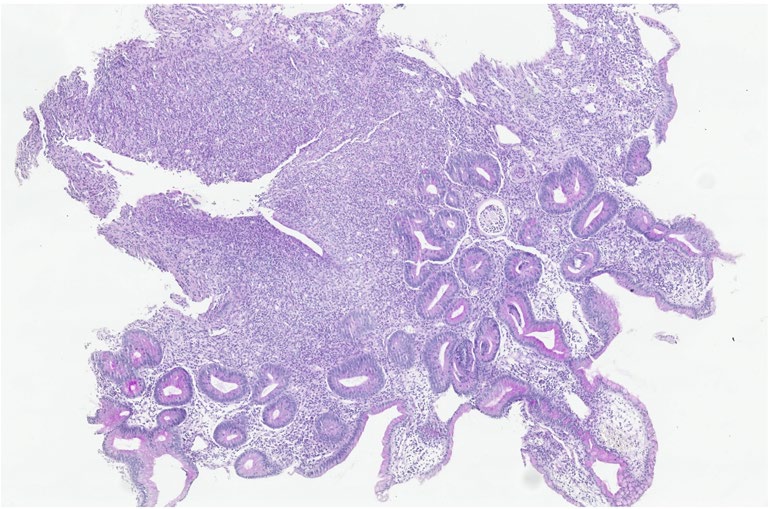
Capecitabine is an oral fluropyrimidine used as palliative
chemotherapy in colorectal and breast cancer patients. Diarrhea is a well-known side effect of capecitabine, being ileitis cases rare. The authors present a case of a terminal ileitis as a late iatrogenic effect of capecitabine treatment.
Downloads
References
Nicosia L, Russo I, De Sanctis V, Minniti G, Valeriani M, Osti MF. Two Cases of Capecitabine-Induced Ileitis in Patients Treated with Radiochemotherapy to the Pelvis and Review of the Literature. J Gastrointest Cancer. 2018;49:538-42. doi: 10.1007/s12029-017-9955-4.
Dao AE, Hsu A, Nakshabandi A, Mandaliya R, Nadella S, Sivaraman A,et al. Role of colonoscopy in diagnosis of capecitabine associated
ileitis: Two case reports. World J Gastrointest Endosc. 2019 May 16;11(5):383-388. doi: 10.4253/wjge.v11.i5.383.
Radwan R, Namelo WC, Robinson M, Brewster AE, Williams GL. Ileitis secondary to oral capecitabine treatment? Case Rep Med.
;2012:154981. doi: 10.1155/2012/154981.
van Hellemond IE, Thijs AM, Creemers GJ. Capecitabine-associated terminal ileitis. Case Rep Oncol. 2018;11:654-9. doi:
1159/000492781.
Klimko A, Tieranu CG, Olteanu AO, Preda CM, Ionescu EM. Capecitabina-induced terminal ileitis: Case report and literature review.
Cureus.2021;13:e14621. doi: 10.7759/cureus.14621.
Lam SW, Guchelaar HJ, Boven E. The role of pharmacogenetivs in capecitabine efficacy and toxicity. Cancer Treat Rev. 2016;50:9-22. doi: 10.1016/j.ctrv.2016.08.001.
Lee SF, Chiang CL, Lee AS, Wong FC, Tung SY. Severe ileitis associated with capecitabine: two case reports and review of the literature.
Mol Clin Oncol. 2015, 3:1398-400. doi: 10.3892/mco.2015.635.
Mokrim M, Aftimos PG, Errihani H, Piccart-Gebhart M. Breast cancer, DPYD mutations and capecitabine related ileitis: description
of two cases and a review of the literature. BMJ Case Rep. 2014;2014:bcr2014203647. doi: 10.1136/bcr-2014-203647.
Al-Gahmi AM, Kerr IG, Zekri JM, Zagnoon AA. Capecitabine-induced terminal ileitis. Ann Saudi Med. 2012;32:661-2. doi: 10.5144/0256-
2012.661.
European Medicines Agency. EMA recommendations on DPD testing prior to treatment with fluoracil, capecitabine, tegafur and flucytosine. EMA/229267/2020. [Jan 2023] Available from: https://www.ema.europa. eu/en/news/ema-recommendations-dpd-testing-prior-treatment-fluorouracil-capecitabine-tegafur-flucytosine
Downloads
Published
How to Cite
Issue
Section
Categories
License
Copyright (c) 2024 Carolina Amado, Ana Rocha Oliveira, Bárbara Paracana, Laura Baptista, Gisela Gonçalves, Margarida Cruz

This work is licensed under a Creative Commons Attribution 4.0 International License.






